Papathanasiou Lab
Research Publications Group Members BiographyFunctional consequences of errors in mitosis
Proper division of the genomic material is fundamental for cell homeostasis. Although cells have developed a plethora of mechanisms to ensure error-free division, mistakes during mitosis are common in normal physiology and a hallmark of disease. Such mistakes give birth to imbalanced, aneuploid genomes and contribute to senescence and ageing. Yet, we do not fully understand the effects of imbalanced genomes on cellular function and how these abnormalities contribute to disease initiation and progression.
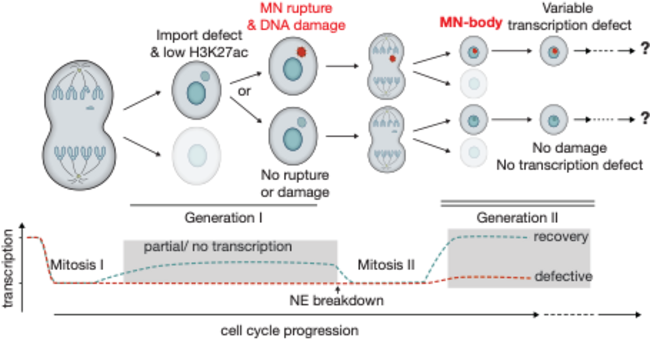
A prominent manifestation of mitotic errors is the generation of abnormal nuclear structures, such as micronuclei and chromosome bridges, which are common features of nuclear atypia in cancer with major physiological significance. Micronuclei (MN, Figure 1) are miniature, additional nuclei that form when a chromosome lags during mitosis and then recruits its own nuclear envelope. These structures accumulate massive DNA damage and can lead to chromosomal rearrangements (e.g. so-called “chromothripsis”) and ongoing genetic instability by mechanisms that are now starting to be understood. We seek to understand how mitotic errors and nuclear abnormalities affect cellular homeostasis from a fresh perspective: the elucidation of non-genetic effects on genome function and cellular adaptation.
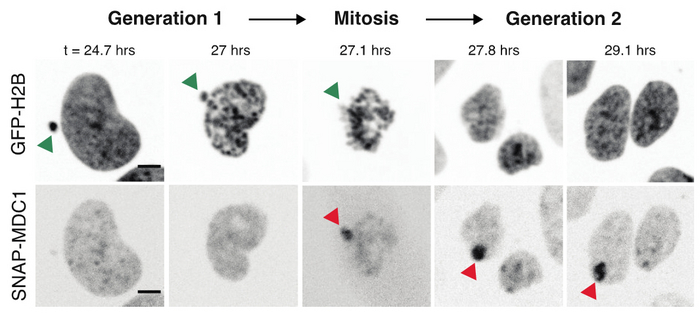
We previously discovered a new functional consequence of mitotic errors by showing that micronuclei are a previously unappreciated source of transcriptional heterogeneity and epigenetic instability. Chromatin alterations are inherited after the micronuclei reincorporate into the normal nuclear environment of daughter cells and are strongly associated with long-lived DNA damage. We termed these structures of reincorporated MN chromosomes with altered chromatin “MN-bodies” (Figures 1 and 2). MN-bodies represent one type of abnormal nuclear structure that may be formed from abnormal chromosomes derived from mitotic errors (“Mit-bodies”).
This discovery, together with the novel technologies that we have developed, will serve as the basis for addressing four fundamental questions on the functional consequences of mitotic errors:
1) How is chromatin state affected in abnormally segregated chromosomes?
2) What are the mechanisms of transcriptional (dys)regulation following errors in mitosis?
3) How is genome organisation perturbed in imbalanced genomes?
4) What is the physiological significance of abnormal chromosomes generated by mitotic errors?
We aim to develop new, innovative approaches and build upon the novel methods we have recently established, such as a new experimental and computational framework that links phenotype to function at the single-cell level (“Look-Seq2”) and advanced cellular systems for long-term tracking of mis-segregated chromosomes.
Recruiting
We are continuously looking for motivated people at all levels to join our team and advance their (and our) knowledge on cool aspects of abnormal cell division. Please send your CV and any thoughts to Stamatis Papathanasiou by email.