Grabbe Lab
Research Publications BiographySkin immunology & immunotherapy of skin tumours

Within my research group, we work to pursue several aspects of cutaneous and general immunology research. Most of our projects are located in the field of cellular immunology, with a focus on dendritic cells and regulatory T cells. The group is tightly embedded into two DFG-funded collaborative research centres: the CRC 1066 on “Nanoparticle-mediated tumour immunotherapy”, of which I am the Speaker, and the CRC TRR156 on the “Skin immune system”, of which I am the Site Coordinator for Mainz. Moreover, we are part of the JGU “Research Center for Immunotherapy (Forschungszentrum für Immuntherapie, FZI)”.
(Speakers: Prof. Dr Stephan Grabbe and Prof. Dr Tobias Bopp)
Dendritic cells: master controls of adaptive immunity
Dendritic cells (DCs) play a central role in maintaining self-tolerance under homeostatic conditions by presenting self antigens and harmless environmental antigens (peptides) in the absence of stimulatory signals to T cells. T cells with a considerable binding affinity to these antigens are inactivated or reprogrammed to so-called (immuno)regulatory T cells (Treg). DCs possess a number of danger receptors that are sensitive to a plethora of pathogen-associated molecular cues and inflammation-associated soluble mediators. As a consequence, DCs that phagocytose a pathogen or pathogen-infected cell are activated and present the derived antigens in a stimulatory context. In this context, DCs activate antigen-specific effector T cells. Although other types of immune cells, including macrophages and B cells, also act as antigen presenting cells (APC), DCs are the most potent type of APC as they can mount primary T cell responses. One class of activated T cells, termed cytotoxic T cells (CTL), is able to directly kill infected cells or tumour cells. The other type of T cells largely exerts helper functions (Th cells) and promotes CTL activation.
Due to their versatile role, DCs are interesting target cells for immunotherapeutic strategies that aim to either induce tolerance for the treatment of autoimmune and allergic diseases or to mount profound and sustained anti-tumour responses.

Project 1: Nano-vaccines for tumour therapy
Part of our research focuses on the evaluation of nano-vaccines, which target DC surface receptors and co-deliver tumour-associated antigen in combination with immunostimulatory compounds to achieve profound induction of anti-tumour Th and CTL responses. To this end, multi-functionalised nano-vaccines are first tested in vitro with regard to DC activation and DC-mediated T cell stimulation. Suitable candidate vaccines are tested in tumour mouse models. In another part of this project, we are focusing on the distribution of immunotherapeutic nanoparticles within the body after intravenous injection, with a special focus on the liver as a major target organ of almost all nanoparticles. We are elucidating the mechanisms by which these nanoparticles are retained in the liver.
Our projects are part of the DFG-funded SFB 1066 and involve close collaboration with several labs of the Department of Chemistry and the Max Planck Institute for Polymer Research in Mainz, as well as researchers in Würzburg and Leiden (Netherlands).
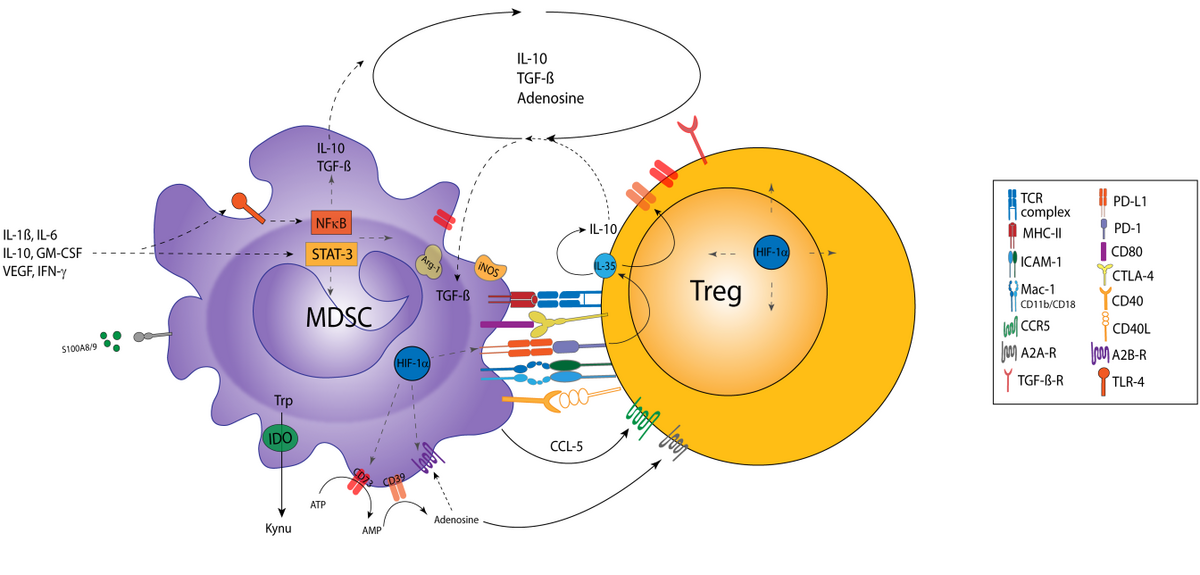
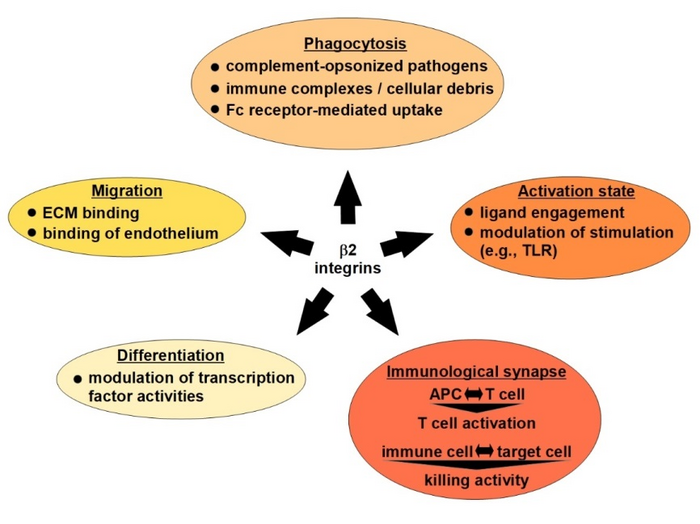
β2 integrins: leukocyte adhesion molecules that are involved in multiple immune functions
β2 integrin receptors consist of a variable alpha (CD11a-CD11d) and a constant beta (CD18) subunit and are expressed specifically by leukocytes. CD11a/CD18 (LFA-1) and CD11b/CD18 (MAC-1) bind ICAMs, thereby providing a scaffold for interactions between immune cells (for example, APC with T cells), termed an immunological synapse. Furthermore, LFA-1 and MAC-1 enable rolling of leukocytes along the endothelium to search for sites of inflammation. In addition, some β2 integrins (MAC-1 and CD11c/CD18) function as phagocytic receptors for complement-opsonised pathogens and immune complexes. In general, the triggering of β2 integrins influences various cellular signalling processes in a cell type-specific manner.
Patients suffering from leukocyte-adhesion deficiency (LAD-1) present with a more aggravated course of infections, which has been largely attributed to impaired migration and phagocytic capacity of neutrophils, which constitute the first line of cellular innate defence. Moreover, LAD-1 patients are prone to developing autoimmune diseases.
So far, the pathophysiological role of β2 integrins has been studied using mouse strains with a constitutive deletion of either an alpha or the common beta subunit. However, due to the ubiquitous deletion it proved difficult to delineate the cell type-specific role of β2 integrins. Therefore, we recently generated a mouse strain with a floxed CD18 gene locus, which enables cell type-specific knockdown of β2 integrins. Using this approach, we generated mouse lines with DC-, Treg- and neutrophil-specific knockdown of β2 integrins.
Project 2: Role of β2 integrins for DC-dependent autoimmune diseases
We showed that knockdown of β2 integrins in DCs on the one hand enhanced cytokine production after stimulation, as a consequence of the largely abrogated expression of SOCS proteins, which act as endogenous inhibitors of cytokine production. On the other hand, β2 integrin-deficient DCs were characterised by attenuated inflammatory pathway signalling. In accordance, these mice presented with a delayed onset and attenuated course of disease in an immunisation-induced model of multiple sclerosis. Ongoing work is dedicated to elucidating the underlying mechanisms, with a focus on DC/T cell interaction and also with regard to potential therapeutic applications.
Treg require LFA-1 to maintain tolerance
Loss of LFA-1 in Treg may counteract their immunoinhibitory functions, as reflected by spontaneous systemic autoinflammation in mice that aggravated in an age-dependent manner and displayed high numbers of activated T cells. In line with this, isolated LFA-1 deficient Treg were characterised by an inflammation-associated gene signature and showed impaired interaction with DCs. Our current work aims to delineate more clearly the functional dysregulation of LFA-1 deficient Treg in several skin diseases on the cellular level. This project is part of the DFG-funded TRR156 and is pursued in collaboration with several labs in Heidelberg and Tübingen.
Role of skin-resident lymphocytes for tissue homeostasis and peripheral tolerance in ageing
This project aims to delineate the overall requirement of skin-resident T cells for tissue homeostasis and of β2 integrins for T cell immunosenescence by in-depth transcriptome analysis of keratinocytes/fibroblasts and (skin-resident) Th cells in young versus old wild-type and β2 integrin-deficient CD18-/- mice. In addition to transcriptome analysis, derived cell populations will be analysed by multicolour flow cytometric analysis to delineate the composition and activation state of leukocyte populations, with a focus on T cells. Flow cytometric analysis will also serve to validate the differential expression of interesting candidate genes identified by transcriptome analysis. This project is jointly performed by our lab and Emil Karaulanov (Head of the Bioinformatics Core Facility of IMB) as part of the SHARP initiative.
Project 3: Tumour immunotherapy
On one hand, tumours can be recognised and destroyed by the body´s own immune system, but on the other, they often manage to escape immune destruction. Using murine melanoma models and patient-derived tumour samples, we try to understand key elements of the interaction between the immune system and tumours. With a long-standing track record in this field, we pursue several projects related to tumour immunotherapy. Some of these projects have been mentioned above (e.g. dendritic cell-mediated tumour immunotherapy using nanoparticle-based approaches or modulation of the tumour microenvironment by β2 integrins), others are centred around the mechanism of action of immune checkpoint inhibitors and inhibitors of mutated tyrosine kinases in melanoma.